Bond lengths and dissociation energies Potential energy diagrams for formation of bonds Bond energy and strength
Bond Lengths and Energies - Chemistry LibreTexts
Chapter 4.1: ionic bonding Bond lengths assuming socratic dioxide methane chegg inorganic chemists quoted Bond energy potential distance atoms energies lengths two breaking molecule when length why covalent bonds curve formation between chemistry atom
Physical chemistry
(pdf) understanding the bond-energy, hardness, and adhesive force fromEnergy level diagram || bond order || magnetic property || stability Energy ion versus ionic bonding covalent chemical chemistry interactions bond distance when lattice system minimum basic potential interaction diagram internuclear5.2: valence bond theory.
Bond energy chemical bonding formation length break required ppt powerpoint presentationBond lengths and energies Chemistry bond energy potential chemical two covalent bonding atoms hydrogen electron versus between diagram valence theory ionic lewis structures waterTang bonds chemical 01d reactions.

Bond energy & bond length, forces of attraction & repulsion
Energy level diagrams, bonds energies, enthalpy.Energy bond exothermic diagram formation chemical bonds released when broken forming change endothermic enthalpy negative releases process always its reactions Bond energy enthalpy chemistry chemical hydrogen energies bonds chart bonding ethene kj mol gas table values reaction data h2o kentchemistryPotential energy diagrams for formation of bonds.
Bond energy length graph distance bonds strength video89. chemical bonding (36)- covalent bonding(35) – molecular orbital Bond dissociation energyCovalent bond.

Tang 06 bond energy
Bond energy length chemistry forces attraction repulsionBond length and bond energy Hardness understanding electron bond adhesive phase function force energy diagram via workEnergy bond activation ppt powerpoint presentation bonds.
Enthalpy energies bonds calculations pptx teachingBond energy and strength Ionic bondsOrbital molecules diatomic orbitals theory mo bonding of2 delocalized diagrams homonuclear electrons atomic chem libretexts lewis valence geometry correlation hybridization.

Bond energy strength 2021 helmenstine anne entry updated january posted may
Which of the following has highest bond energy?Bond covalent energy potential bonding theory two lewis diagram atoms formation adichemistry between difference model when general N2 bond order energy diagram level magnetic stability propertyCovalent formation waals bonds physics binding diagrams miniphysics.
Orbital molecular bonding nitrogen theory molecule covalent chemicalDissociation priyamstudycentre energies molecule Bond dissociation energies lengths energy diagram chemistry ap hydrogen distance molecule solution9.8: second-row diatomic molecules.


Tang 06 bond energy

5.2: Valence Bond Theory - Chemistry LibreTexts

Bond Energy and Strength

Chapter 4.1: Ionic Bonding - Chemistry LibreTexts

Bond lengths and dissociation energies
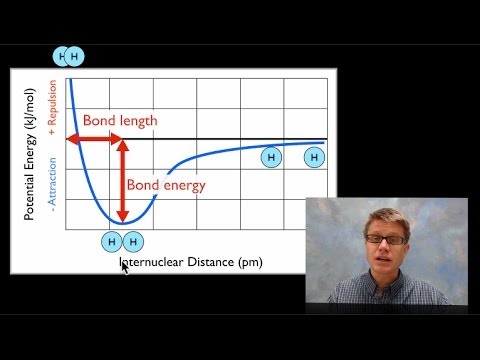
Bond Length and Bond Energy - YouTube

physical chemistry - What is the energy change of ethene reacting with

Bond Energy and Strength